Category: Science
Nearly 100,000 Americans Estimated to be Diagnosed with Melanoma in 2023
By Joan Levy, PhD - MRA Chief Science Officer | 19 January 2023 In News, Prevention, Science, Treatment
Each January, the American Cancer Society (ACS) releases updated estimates about trends in new cancer cases and deaths in its annual report, Cancer Facts and Figures. This report highlights the estimated incidence (number of new cases), prevalence (number of people alive today with a history of cancer), and survival statistics for cancer in the United States.
Registration Now Open: MRA's 2023 Patient Forum
19 January 2023 In Allies & Partnerships, Events, News, Prevention, Science, Treatment
Registration for MRA's Melanoma Exchange Patient Forum, held in-person in Washington DC and virtually on Wednesday, March 8, is now open. Register now to hear from leading experts in the melanoma field and have the opportunity to ask questions, discuss, and network with other people who are going through their own melanoma journey.
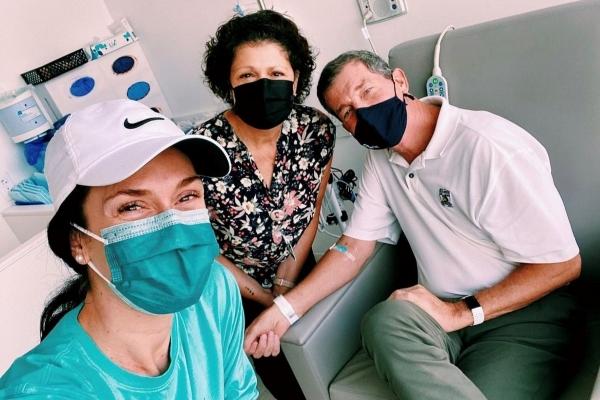
From Melanoma Patient to Caregiver
By Renee Orcione, MRA Digital Engagement & Communications Manager | 11 November 2022 In Allies & Partnerships, Melanoma Stories, Prevention, Science, Treatment
By the time she was 26-years old, Leah Adams had spent countless hours tanning. Growing up in Ohio where the winters were long, she felt a pressure to stay tan far beyond the summer months, so she turned to tanning beds.
FDA Approves Opzulera for At-Home Therapy for Skin Repigmentation: What this Means for Melanoma Patients with Vitiligo
By Isabel Ryan, MRA Intern | 24 October 2022 In News, Science
On July 18th, the U.S. Food & Drug Administration (FDA) approved the first topically-applied treatment for skin repigmentation for patients with vitiligo after strong results from two Phase 3 trials. Vitiligo, a chronic skin disease, affects 3 in 100 people who have melanoma, categorized as melanoma-associated vitiligo. The approval invites patients with vitiligo, including those with melanoma, to talk with their providers on how Opzelura can serve their skin needs.
Overcoming Melanoma Treatment Resistance Research Update
24 August 2022 In Science, Treatment
About half of all patients given cancer immunotherapies do not respond to them, and although targeted therapies often dramatically shrink tumors in most patients, in about three-quarters of those patients, the treatments stop being effective weeks to months after initially killing tumor cells.
Improving AI Performance for People of Color: Diagnosing Melanoma & Other Skin Cancers
By Marc Hurlbert, PhD, MRA Chief Executive Officer | 12 August 2022 In Allies & Partnerships, Prevention, Science
The problem with the current state-of-the-art AI and image-based algorithms is that they have been developed using images of moles from light skinned (white) individuals. As a result, existing AI tools are not sensitive enough in people with darker skin. Dr. Albert Chiou, Clinical Associate Professor of Dermatology at Stanford University, the junior faculty member on the L’Oréal Dermatological Beauty Brands-MRA Team Science Award, and his colleagues are working to fix these shortcomings.
MRA's Latest Research Report: The Melanoma Research Community Reunites
11 August 2022 In Allies & Partnerships, Events, Melanoma Stories, News, Prevention, Science, Treatment
Each year, MRA brings together hundreds of people from across the melanoma research community to exchange ideas, report on scientific progress, celebrate achievements, and mourn our collective losses.
Melanoma Clinical Trial Success Story: From Patient Volunteer to No Evidence of Disease
By Renee Orcione, MRA Digital Engagement & Communications Manager | 3 August 2022 In Melanoma Stories, Science
This Mom of three enrolled in a neoadjuvant clinical trial for Stage 3 melanoma. After two infusions of Opdualag, Shannon underwent a successful surgery where doctors removed all affected lymph nodes and tested them, revealing No Evidence of Disease (NED).
What Uveal Melanoma Patients Need to Know about KIMMTRAK
By Cody Barnett, MPH, MRA Senior Director of Communications & Patient Engagement | 18 July 2022 In Science, Treatment
You may have heard of KIMMTRAK, a new therapy that recently earned FDA approval for the treatment of metastatic or unresectable uveal melanoma. Uveal melanoma, also called ocular melanoma, is a rare melanoma subtype that affects about 2,500 people each year in the United States. KIMMTRAK is the first drug to earn FDA approval to treat these patients.
New Funding Alert: Department of Defense Melanoma Research Program
1 July 2022 In Allies & Partnerships, News, Science
Research Funding Alert: The Department of Defense Melanoma Research Program will award up to $40 million in funding for melanoma research in FY22. Learn more about funding mechanisms, focus area, and deadlines!